A Geologists View of the Enhanced Greenhouse Effect
What is the Greenhouse Effect?
 The simplest way to think of the greenhouse effect is this – gases in the Earth’s atmosphere absorb some of the heat that is escaping from the Earth into space and then re-radiate that heat. About half gets radiated back towards the Earth. This extra heat makes the lower atmosphere and the surface of the Earth warmer than it would otherwise be.
The simplest way to think of the greenhouse effect is this – gases in the Earth’s atmosphere absorb some of the heat that is escaping from the Earth into space and then re-radiate that heat. About half gets radiated back towards the Earth. This extra heat makes the lower atmosphere and the surface of the Earth warmer than it would otherwise be.One of the gases that causes the greenhouse effect is carbon dioxide. This exists naturally on the Earth and is known to have been part of the atmosphere since the Earth has had one. The amount has, however, significantly varied over time. The original CO2 came from volcanic activity. Volcanoes are still adding carbon dioxide to the atmosphere today – as is the process of respiration which all organisms perform. The level of natural CO2 in the atmosphere is about 280ppm (parts per million). This amount of CO2 keeps the Earth warmer than it would be without it, without this natural CO2 the Earth would be too cold for complex life to have successfully developed.
What is the Enhanced Greenhouse Effect?
Carbon dioxide levels in the atmosphere are higher than they would naturally be. This is no surprise, as we know that humans have been adding, significantly, to the levels of carbon dioxide in the atmosphere since the start of the industrial revolution. In a study completed in 2009, researchers reconstructed atmospheric carbon dioxide levels over the past 2.1 million years in the sharpest detail yet.
In the study, geochemists reconstructed CO2 levels by analyzing the shells of single-celled plankton buried under the Atlantic Ocean. By dating the shells and using new techniques to look at their chemistry, they were able to estimate how much CO2 was in the air when the plankton were alive. They showed that peak CO2 levels over the last 2.1 million years averaged only 280 parts per million; but in 2009 CO2 was at 385 parts per million, or 38% higher.
 |
| Plankton - Water Dwelling, microscopic organisms |
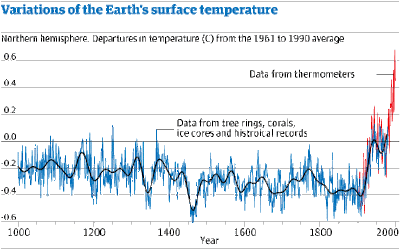
This increase in carbon dioxide levels has been accompanied by an increase in global temperatures – this extra temperature rise caused by the excess of carbon dioxide is called the Enhanced Greenhouse Effect (EGE). The graph below shows this variation. There is a distinct temperature rise from the time of the industrial revolution until now.
More Evidence – Looking at Ice Cores
Geochemists have been able to use air trapped in the ice of the Antarctic to look at carbon dioxide levels in the atmosphere over the past 800,000 years. The technique depends on the idea that a new layer of snow ice used to build up on the Antarctic surface every year. The snow layers contain some air trapped with the ice. As the snow layers build up the ones below get buried and some of the air is trapped in the buried layers. By taking an ice core it is therefore possible to ‘drill down’ through the snow ice of many years and, by carefully extracting the air from the samples, it is possible to see how much carbon dioxide has been in the air at different times. The graph below shows what the geochemists have found for air samples from the past 2000 years. This graph also shows the concentrations of two other greenhouse effect causing gases, methane (which has a far greater effect) and nitrogen oxide.
It can be seen from the graph that the atmospheric concentrations of all these gases stayed about the same until the start of the industrial revolution, since when they have all risen dramatically. There would appear to be a demonstrated link between the temperature rises of the Enhanced Greenhouse Effect and the atmospheric concentration of these greenhouse gases.
The Effect on the Earth’s Ice – and why the EGE will get worse
If the temperature of ice rises above 0oC it is likely to melt – we all know this. For this to occur a large mass of ice needs to rise above 0oC and so it’s quite hard to achieve. Those arguing against the existence of the EGE are quick to point out that the EGE could not achieve this - which is largely correct, but irrelevant!
Ice can also sublime (which means turn straight into a vapour). This can happen when the surface of the ice gets an extra bit of energy, and this will naturally occur as a result of the increase in temperature from the EGE. The graph below shows that solid water can turn into vapour at temperatures well below 0oC.
The EGE appears to have caused enough of a temperature rise to cause enough ice to sublime over significant parts of the Earth’s surface. The evidence from the shrinking of the Rhone glacier in Switzerland has become famous, the picture here shows the glacier in 1870 (on the postcard) and 2010. There has clearly been a massive reduction in the amount of ice in the glacier – it has shrunk back 3km!
Far worse than this, however, is the effect of the EGE on the polar ice. The photos below show the difference between the amount of ice the developed at the North Pole (the Arctic) in 1979 and 2003.
There was noticeably less of the ‘white stuff’ in 2003. This is serious – not because it will cause sea level rise (it won’t, sea ice doesn’t add to or reduce sea levels) but because the white ice should be reflecting the Sun’s heat back into space. This ability to reflect is called the ‘albedo’. As less ice is now forming, the Earth’s albedo is getting lower. That means less heat will get reflected back into space, which means that the Earth will get even hotter. This type of process, where an effect of the process causes something to happen that, in turn, makes the original process happen more is called a feedback loop. They accelerate very quickly. There are those that say we may already be too late to stop the onset of the sixth mass extinction! They could be right...
If the Earth's poles were not covered in ice then the mechanism that puts oxygen into the deep oceans would be lost. At present, when ocean currents cause sea water to collide with the polar ice it gets cold. This makes it more dense than the surrounding water so it sinks. As the water was near the surface of the ocean it was dissolving oxygen as it went. When the water falls it takes this oxygen into the deep oceans where it can sustain life. If we lose this mechanism then the life at depth will die. That will lead to the production of hydrogen sulphide gas as the organisms rot. The hydrogen sulphide will poison other lifeforms - this will be another example of a feedback lop. When there is too much hydrogen sulphide for the oceans to dissolve it will start to leak out into the atmosphere and wipe out the life from the land as well. This is why we could be headed for the sixth mass extinction.
For more detailed information on climate change and its effect on the oceans click here .




No comments:
Post a Comment