PCSA Geology Updates
Support and revision notes for GCSE Geology
Monday, 8 October 2012
Friday, 11 May 2012
Exam Technique...
A little list of tips to help you make the best use of what you know in the exam...
Remember - reading the questions and looking at the diagrams carefully will help you to get more marks.
Don't be frightened off by bits of questions that look hard - do the bits you can (there normally will be some on every question no matter how hard it looks).
Work steadily - you have 90 minutes to get 100 marks so there's no need to rush.
USE the geology words that you know - they get you extra marks.
Look at the number of marks availbale. On 1 and 2 mark questions answer with that number of facts. On 3 - 5 mark questions give one more fact than there are marks for the question (this gives the examiner another chance to give you the marks).
I'd say good luck - but if you've thoroughly prepared for the exam you won't need it!
So - may your success rise like an anticline and may al your faults be breaks in the rock (normal, reverse or thrust)!
DP
A little list of tips to help you make the best use of what you know in the exam...
Remember - reading the questions and looking at the diagrams carefully will help you to get more marks.
Don't be frightened off by bits of questions that look hard - do the bits you can (there normally will be some on every question no matter how hard it looks).
Work steadily - you have 90 minutes to get 100 marks so there's no need to rush.
USE the geology words that you know - they get you extra marks.
Look at the number of marks availbale. On 1 and 2 mark questions answer with that number of facts. On 3 - 5 mark questions give one more fact than there are marks for the question (this gives the examiner another chance to give you the marks).
I'd say good luck - but if you've thoroughly prepared for the exam you won't need it!
So - may your success rise like an anticline and may al your faults be breaks in the rock (normal, reverse or thrust)!
DP
Thursday, 22 December 2011
The Enhanced Greenhouse Effect
A Geologists View of the Enhanced Greenhouse Effect
What is the Greenhouse Effect?
 The simplest way to think of the greenhouse effect is this – gases in the Earth’s atmosphere absorb some of the heat that is escaping from the Earth into space and then re-radiate that heat. About half gets radiated back towards the Earth. This extra heat makes the lower atmosphere and the surface of the Earth warmer than it would otherwise be.
The simplest way to think of the greenhouse effect is this – gases in the Earth’s atmosphere absorb some of the heat that is escaping from the Earth into space and then re-radiate that heat. About half gets radiated back towards the Earth. This extra heat makes the lower atmosphere and the surface of the Earth warmer than it would otherwise be.One of the gases that causes the greenhouse effect is carbon dioxide. This exists naturally on the Earth and is known to have been part of the atmosphere since the Earth has had one. The amount has, however, significantly varied over time. The original CO2 came from volcanic activity. Volcanoes are still adding carbon dioxide to the atmosphere today – as is the process of respiration which all organisms perform. The level of natural CO2 in the atmosphere is about 280ppm (parts per million). This amount of CO2 keeps the Earth warmer than it would be without it, without this natural CO2 the Earth would be too cold for complex life to have successfully developed.
What is the Enhanced Greenhouse Effect?
Carbon dioxide levels in the atmosphere are higher than they would naturally be. This is no surprise, as we know that humans have been adding, significantly, to the levels of carbon dioxide in the atmosphere since the start of the industrial revolution. In a study completed in 2009, researchers reconstructed atmospheric carbon dioxide levels over the past 2.1 million years in the sharpest detail yet.
In the study, geochemists reconstructed CO2 levels by analyzing the shells of single-celled plankton buried under the Atlantic Ocean. By dating the shells and using new techniques to look at their chemistry, they were able to estimate how much CO2 was in the air when the plankton were alive. They showed that peak CO2 levels over the last 2.1 million years averaged only 280 parts per million; but in 2009 CO2 was at 385 parts per million, or 38% higher.
 |
| Plankton - Water Dwelling, microscopic organisms |
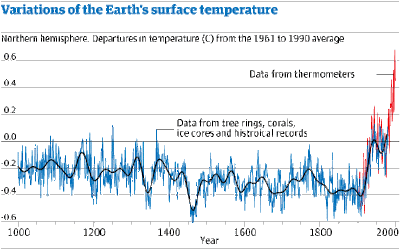
This increase in carbon dioxide levels has been accompanied by an increase in global temperatures – this extra temperature rise caused by the excess of carbon dioxide is called the Enhanced Greenhouse Effect (EGE). The graph below shows this variation. There is a distinct temperature rise from the time of the industrial revolution until now.
More Evidence – Looking at Ice Cores
Geochemists have been able to use air trapped in the ice of the Antarctic to look at carbon dioxide levels in the atmosphere over the past 800,000 years. The technique depends on the idea that a new layer of snow ice used to build up on the Antarctic surface every year. The snow layers contain some air trapped with the ice. As the snow layers build up the ones below get buried and some of the air is trapped in the buried layers. By taking an ice core it is therefore possible to ‘drill down’ through the snow ice of many years and, by carefully extracting the air from the samples, it is possible to see how much carbon dioxide has been in the air at different times. The graph below shows what the geochemists have found for air samples from the past 2000 years. This graph also shows the concentrations of two other greenhouse effect causing gases, methane (which has a far greater effect) and nitrogen oxide.
It can be seen from the graph that the atmospheric concentrations of all these gases stayed about the same until the start of the industrial revolution, since when they have all risen dramatically. There would appear to be a demonstrated link between the temperature rises of the Enhanced Greenhouse Effect and the atmospheric concentration of these greenhouse gases.
The Effect on the Earth’s Ice – and why the EGE will get worse
If the temperature of ice rises above 0oC it is likely to melt – we all know this. For this to occur a large mass of ice needs to rise above 0oC and so it’s quite hard to achieve. Those arguing against the existence of the EGE are quick to point out that the EGE could not achieve this - which is largely correct, but irrelevant!
Ice can also sublime (which means turn straight into a vapour). This can happen when the surface of the ice gets an extra bit of energy, and this will naturally occur as a result of the increase in temperature from the EGE. The graph below shows that solid water can turn into vapour at temperatures well below 0oC.
The EGE appears to have caused enough of a temperature rise to cause enough ice to sublime over significant parts of the Earth’s surface. The evidence from the shrinking of the Rhone glacier in Switzerland has become famous, the picture here shows the glacier in 1870 (on the postcard) and 2010. There has clearly been a massive reduction in the amount of ice in the glacier – it has shrunk back 3km!
Far worse than this, however, is the effect of the EGE on the polar ice. The photos below show the difference between the amount of ice the developed at the North Pole (the Arctic) in 1979 and 2003.
There was noticeably less of the ‘white stuff’ in 2003. This is serious – not because it will cause sea level rise (it won’t, sea ice doesn’t add to or reduce sea levels) but because the white ice should be reflecting the Sun’s heat back into space. This ability to reflect is called the ‘albedo’. As less ice is now forming, the Earth’s albedo is getting lower. That means less heat will get reflected back into space, which means that the Earth will get even hotter. This type of process, where an effect of the process causes something to happen that, in turn, makes the original process happen more is called a feedback loop. They accelerate very quickly. There are those that say we may already be too late to stop the onset of the sixth mass extinction! They could be right...
If the Earth's poles were not covered in ice then the mechanism that puts oxygen into the deep oceans would be lost. At present, when ocean currents cause sea water to collide with the polar ice it gets cold. This makes it more dense than the surrounding water so it sinks. As the water was near the surface of the ocean it was dissolving oxygen as it went. When the water falls it takes this oxygen into the deep oceans where it can sustain life. If we lose this mechanism then the life at depth will die. That will lead to the production of hydrogen sulphide gas as the organisms rot. The hydrogen sulphide will poison other lifeforms - this will be another example of a feedback lop. When there is too much hydrogen sulphide for the oceans to dissolve it will start to leak out into the atmosphere and wipe out the life from the land as well. This is why we could be headed for the sixth mass extinction.
For more detailed information on climate change and its effect on the oceans click here .
Wednesday, 21 December 2011
Environmental Geoscience
We produce vast amounts of waste. Much of this waste ends up in landfill sites. Almost all landfill sites are old quarries. One of the tasks of an environmental geoscientist could be to decide whether an old quarry is suitable for use as a landfill site.
What the Syllabus Says - and the Details
Environmental geologists study:
Planning for and monitoring of waste disposal...
Some of the rubbish placed into a landfill site will steadily rot away. As it does so it will produce flammable gases such as methane, toxic (poisonous) gases, and various chemicals that will dissolve in water. So - easy job - just stop the site catching fire or poisoning the local environment!
You'll need to identify a site of suitable size where the rock is impermeable (to stop polluted water flowing into the ground). Your site will need suitable road access - check the rock under the road is strong enough to deal with all the heavy lorries arriving.
Monitoring of potentially polluted water - get your geochemists to check the chemical content of the water in the ground and in the local streams on a regular basis. If you're poisoning the water then you're poisoning farmland, and possibly the local water supply!
Restoration of polluted soils - Landfill sites, quarries and spoil heaps from mines can all end up polluting the water that flows through them and this will pollute the soils where that water then flows to. Whilst you may need to construct concrete barriers in the ground to prevent this flow while the industry is happening, there will come a time when it finishes. At this point you will need to test the soil to see how polluted it is - and quite possibly entirely cover the area with new!
The Geology of Water Supply and Building Things
Water is vital to human life. Early settlements developed along rivers and other places where water was easily avalable. Now that we have a far larger population, and reasons to live in places where there isn't an easy supply of water, we have to be able to find it, collect it and get it to where it is needed.
What the Syllabus Says - and the Details
Hydrogeologists study the geological factors affect the siting of reservoirs and dams.
 |
| The reservoir is the lake, the dam is the wall holding the water back. |
These factors are..
Permeability of bedrock - if the rock is permeable because it is porous or has joints through it then the water will flow away through the rock. Not much point building a dam then! Metamorphic rock are generally good for putting reservoirs on as they are highly compacted.
Stability of bedrock - if the rock you're building the dam on isn't stable then the dam will move. Big flood! Bad! Very bad - lots of compensation claims to pay. Metamorphic rocks tend to be stable as they are normally very strong.
Dip of strata - take your inclinometer and go measure the dip...
If the rocks are dipping from the reservoir to the dam - that's bad. Don't build the dam. The weight of water will push it down the dip of the rocks so the dam basically slides away. More floods! More compensation claims! If the rock dips from the dam towards the reservoir - that's good. The weight of the water will only push the dam harder into the bedrock.
Faults - if the faults are under the reservoir they'll only matter if they provide a route for water to flow away through. If the faults are under the dam then they could be forced to move by the weight above - oops!
Joints - these are a pain. (Joint-pain?) Under the reservoir they let the water out. under a dam they would cause the rock to be to weak so the dam could move and collapse.
Geotechnical geologists also need to consider these features when; designing the foundations for buildings, deciding how steep to make a road or railway cutting, or when designing a tunnel.
Dipping rock layers can slide off each other! Rocks with joints can collapse under an increase in load. If the structure of the rock means that it is likely to move when it is loaded with a building then they will ask for designs with 'ties' that attach beds of rock together. With tunnels the problem is that beds might move when you make the hole. If the beds slide into the tunnel you won't have a tunnel anymore.
If the rocks are permeable then that can cause tunnels to fill up with water - they would need to design a concrete liner to keep the water out. Water flowing through permeable rocks can cause cutting walls to slide and collapse.
Dams and reservoirs are expensive - even when they work. It's often better to find a suitable aquifer (a porous or permeable rock, situated below the level of the water table so the spaces in it will be flooded, that we can pump water out of). If the aquifer rock outcrops on the surface
then it will fill up when it rains - water will drain down into it. Notice that the aquifer needs to be between two impermeable layers of rock; these are called aquitards or confining beds.
If the surface outcrop is above where we want to remove the water from then we may be able to just dig a well and the pressure of the water will force the water to rise up it.
If you're really lucky, your aquifer will outcrop at a lower point than where it is filling up. If this happens you'll have the cheapest water source of all - a spring. The aquifer leaks its water onto the surface.
Of course - if you take too much water out of the aquifer it may collapse. That will cause all sorts of problems...
Mining and Mineral Prospecting
In everyday life we depend on copper for electrical wiring, iron for the various steel objects we use, aluminium for wrapping and a range of other metals. All of these have to be mined - and we need to know where to look for them.
What the Syllabus Says - and the Details
You need to be able to interpret geochemical and magnetic prospecting data to identify possible metalliferous mineral resources.
Geochemical Surveying
Metals form compounds with other elements as they react over time - and geological time gives them plenty of opportunity. Many of these compounds will dissolve and so small amounts of them can be carried away by rivers and water flowing through the ground. Geochemists try to find deposits of these chemicals in the sediments at the bottom of rivers and in the soil where water has flowed through.
If they can find traces of the metals in fairly large amounts, that suggests that there is an ore deposit (an accumulation of a useful metallic mineral in the ground) nearby. The key is to look for anomalies - places where the amount of metal content is higher than in the surroundings. The anomaly map below shows accumulations of lead around Denver State in the USA - the interesting bits are the red areas.
The survey won't be cheap to do. You'll only want to look where the geology suggests that there could be ore deposits. Around the edges of plutons is often a good place. The maps below show the results of looking for gold (Au) and tin (Sn) around the southern edge of Dartmoor.
Are you more interested in buying the purple areas or the land which is 'uphill' from them?
Magnetic Surveying
Magnetic surveying only works if you're looking for a magnetic metal; iron, nickel or cobalt. Iron is generally found in beds of iron-rich sandstone, nickel and cobalt in basalt dyke structures or in gabbro plutons. It is common to do the survey from the air, as shown here.
Notice how the magnetometer picks up an increase in the magnetic field strength when it is over the deposit and also detects a fall off in magnetic field to below background levels to either side of the deposit. This happens because the magnetic metal effectively focuses the Earth's magnetic field through it leaving less to go through each side.
The map below shows a magnetic field strength survey of most of Afghanistan. The bits with the highest anomalies are likely to contain the most magnetic metal ore. Of course - you may
have some difficulty as a western world mining company trying to extract what you find!
Oil and Gas Prospecting
Have you learned the section on how oil and gas are made and trapped? You need that information before any of this will make much sense..!
What the Syllabus Says - and the Details
There are technological difficulties and environmental issues involved in exploring for and extracting oil and natural gas. This makes oil exploration difficult and expensive - as the environments you're likely to be hunting for oil and gas in include; sandy deserts of Iraq, icy deserts of Alaska, stormy seas of the North Sea and hot humidity of the Niger delta in Africa - the equipment has to be built to withstand the conditions or it stops working and you make no money. You'll also have problems with wildlife habitats and unfriendly political regimes.
 |
| Roughish conditions in the North Sea |
Geologists prospecting for new reserves use a variety of techniques including mapping and making cross sections. You need to be able to interpret data from these maps and cross-sections to identify possible gas/oilfields (on-shore and off-shore).
First - MAPS
In the work on traps we found we will need a porous rock like sandstone as a reservoir rock and an impermeable one such as shale or salt to act as a cap rock. We'll also need evidence that there could be trap structure such as a fault or an anticline. If the map shows all of these things - buy the mineral rights to the area!
The simplified map below contains the right elements. What are the key bits?
Is this bit of Poland any use..?
...The symmetrical structure shows a fold with the oldest rocks in the middle - so this is an anticline.
...There are sandstones (good reservoir rocks) and shales (good cap rocks).
...There is shale on top of the sandstone - so there could be an anticline trap here.
In fact this is a very good - it's the Bobrka oil field from which over a million tons of oil have been removed since its discovery in 1937.
Second - CROSS SECTIONS
The cross section below is more complicated than you would get on the exam but it shows various traps. Find an anticline trap. Find a fault trap.
The cross section below is more the right level - rather easier!
Geologists will also use geophysical [seismic] techniques. You need to be able to interpret data from seismic surveys to identify possible gas/oilfields. These tend to look like lots of grey lines to start with - so it helps to know what you're looking at...
The seismic survey is done by making a series of very loud bangs, using compressed air if you're at sea or explosives if you're on land. The sound waves which are made reflect off the rock layers in the ground and are detected by hydrophones (at sea) or geophones (on land).
 |
| Seismic Survey at Sea |
The information from the geophones (or hydrophones) is then fed to a computer which uses the time delay between when the 'bang' was made to when the 'echo' was detected to work out how deep the sound wave must have gone into the rocks. Echoes come from the boundaries between rock types so we can use the information to build up a picture of where these boundaries are underground.
Click here for a nice animation showing this happening.
The diagram below is typical of what these look like - the strong reflection lines (heavy black bits) show where the rock boundaries are.
The seismograph above wouldn't get oil exploration geophysicists very excited - no obvious trap features are shown. The ones below would!
 |
| This is the seismic survey data that produced the complicated cross section of the Bremer Oil Field shown above - the fault raps are clearly seen. |
 |
| Seismic Survey 2 |
Seismic Survey 2 is typical of the sort of thing we are looking for. Once you've seen what to look for on the lower picture, you can see the anticline structure and the two faults on the upper one. This would definitely be an area where drilling a test borehole would be a good idea - assuming that there are sandstones to be reservoirs and suitable cap rocks. Now you just have to deal with the cost, the environmental issues, the technological problems...
How Oil and Gas Reservoirs Develop
The modern world relies on fossil fuels, mostly on oil and gas. We extract these resources from underground reservoirs where they have been 'stored' for millions of years. This section looks at how the oil and gas were made, and at how it gets trapped.
What the Syllabus Says - and the Details
Oil and gas are hydrocarbon mixtures. These hydrocarbons come from the rotting soft tissues of millions upon millions of organisms. Hydrogen and Carbon from these rotting tissues remained together where other elements such as nitrogen and oxygen were mostly lost. The result was a series of compounds made of 'only hydrogen and carbon bonded together' - the definition of a hydrocarbon. 'Gas', as we call it, is actually a mixture of several gases; methane (pictured below), ethane, ethene, butane, butene...
 |
| Methane - the dark atom is carbon, the white ones are hydrogen |
The oil is a mixture of heavier hydrocarbons such as octane (shown below) and decane.
 |
| Octane - 8 black carbons, 18 white hydrogens |
Oil and gas are most frequently made in mudstone deposits where the sediment includes very little oxygen, which is why the carbon and oxygen don't form carbon dioxide and water which is normal when organisms rot.
The gas and oil then 'migrates' upwards through the rocks because it is lighter (less dense) than the water in the rocks so it floats up on top of it. The oil and gas can only migrate through porous or permeable rock, so when it rises up so far that it reaches an impermeable layer it can't carry on upwards. When this happens the oil and gas will try to migrate sideways. If there are impermeable rocks to the sides as well then it is trapped. This is good as it means it is now trapped underground and we can find it and drill a hole to put a pipe through so we can get it.
If the oil and gas can migrate through the rock without meeting an impermeable barrier then it will escape from the rocks completely. Oil and gas will only be found trapped where there is a source rock, a porous reservoir rock for the oil and gas to be in (shown blue in the diagram), and an impermeable cap rock (shown yellow) to trap the oil and gas.
 |
| A typical scenario for trapping oil and gas. |
The accumulation of oil and gas by migration from source beds to reservoir rocks depends upon the contrasting porosity and permeability of reservoir and cap rocks. The reservoir rock has to be porous to allow the oil and gas to collect in it. The cap rock has to be impermeable to stop the oil and gas continuing upwards.
These structures that catch oil and gas in this was are called traps. Three of the main types of trap for oil and gas are: anticline traps (top left), fault traps (top right) and unconformity traps (bottom left). In each case the oil (shown in pink) and gas (shown in yellow) are held in the porous reservoir rock, such as sandstone, underneath an impermeable cap rock, such as shale.
The fourth type of trap you have to know about is a salt dome trap - shown below...
 |
| A salt dome trap |
In this situation the oil (grey) is held below an impermeable cap rock (purplish-grey) and can't move sideways because it is against the impermeable barrier of the salt (white). You'll look at how salt domes form in A level Geology and beyond. Salt layers also make excellent cap rocks in anticline and fault traps.
Task - how has the oil got trapped in each of the scenarios shown in the diagram below?
Subscribe to:
Comments (Atom)